Investigating how UTIs recur with organoid and organ-on-a-chip technology
Two new studies utilize novel organoid and organ-on-a-chip technology to investigate antibiotic resistance and urinary tract infection (UTI) recurrence.
Two new tissue models have been developed to help researchers investigate UTIs in the bladder. The models provide a new insight into the behaviors of the causative bacteria, Escherichia coli (E. coli), that help infer them with antibiotic resistance, leading to UTI recurrence. The models were developed by researchers at the École polytechnique fédérale de Lausanne (EFPL; Switzerland) as part of the AntiResist consortium, which aims to deliver more representative in vitro disease models for the development of optimal treatment strategies.
What causes a UTI?
UTIs are typically caused by a subspecies of bacteria known as Uropathogenic E. coli (UPEC). These bacteria infect and multiply in the outer cells of bladder epithelium known as umbrella cells. Once in place, these bacteria can form intracellular bacterial communities, which repeatedly rupture and reinfect bordering cells, leading to a reduction in the umbrella cells present and allowing further penetration into the lining of the bladder.
Why do UTIs recur?
Once buried deeper into the bladder epithelium, UPEC can establish quiescent intracellular reservoirs of bacteria. The bacteria in these reservoirs cease to proliferate and are protected from antibiotic attack, seemingly due to their location deep in the epithelial wall and quiescent cellular state.
These reservoirs can lay dormant during a course of antibiotics. When the immature bladder cells that the reservoirs preside in mature, rising to the surface of the epithelium, UPEC can then reinitiate growth, rupturing into the bladder lumen and leading to a secondary active infection [1].
Investigating UTIs
While there are indications for how these patterns occur, detailed examination of these mechanisms is challenging. Lead author of both studies, Kunal Sharma (EFPL), explained that “infection dynamics are difficult to capture from static imaging of tissue explants at serial time points. Thus far, in vitro models have not recapitulated bladder architecture with sufficient fidelity to study the time course of these events.”
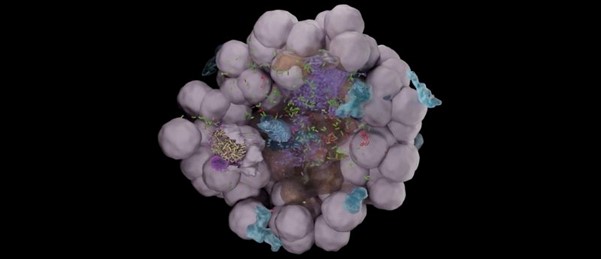
To improve the models available to researchers and to answer their own questions about UTI infections, the team developed two in vitro models: a bladder organoid to accurately represent the tissue structure [2], and a bladder-on-a-chip to replicate the functional, physiological conditions of a bladder [3].
Advanced cell cultures represent a new window through which researchers can observe the developmental biology of organs, the underlying pathogenesis and progression of diseases and the impact of therapeutics on both diseased and healthy tissues.
Applying the organoids
The organoid models were developed with fluorescence-labeled cell membranes, allowing the researchers to analyze them using live-cell confocal imaging. Using this method, the research team identified specific bacterial niches that developed within the models. Observations of multiple organoids revealed various patterns in host pathogen interactions that led to disparate outcomes for the infection.
Further analysis using volumetric electron microscopy, revealed that individual UPEC are able to rapidly infiltrate deep into the layers of the bladder epithelium, bypassing the need to form intracellular bacterial communities. These lone bacteria are, again, protected from antibiotics and immune cells due to their isolated position.
“This proof-of-concept system has shown promising potential for follow up studies on bacterial persistence to antibiotics and the dynamics of immune cell responses to infection,” remarked Sharma.
Bladder-on-a-chip insights
The bladder-on-a-chip models constituted human umbrella and bladder epithelial cells cultured together with a representative urine-flow system. Mechanical stresses akin to those experienced when a bladder fills and is emptied were applied to the model as the researchers examined the growth dynamics of UPEC over time.
This study revealed that neutrophils, recruited to the site of an infection, are incapable of eradicating intracellular bacterial communities. These same communities were found to be highly resistant to successive cycles of antibiotic treatment administered within the model.
Highlighting the utility of these models for investigating UTI recurrence and developing treatment strategies, senior author of both studies Vivek Thacker (EFPL) stated that “the two models complement each other well and are tailored to study specific aspects of the disease. We hope they will serve as a resource for the wider microbiology community and advance the synergies between the tissue engineering and infectious diseases communities.”