Shapeshift, eat brains, disclose evolutionary secrets: a day in the life of an amoeba
A unicellular organism that can rebuild itself into a different organism, may also be a crawling, swimming archive of evolutionary knowledge. When it’s not eating your brain.
There’s a strange irony to the fact that a close relative of the brain-devouring amoeba species, Naegleria fowleri, could be the source of a greater understanding of the fundamental rules underpinning life on Earth.
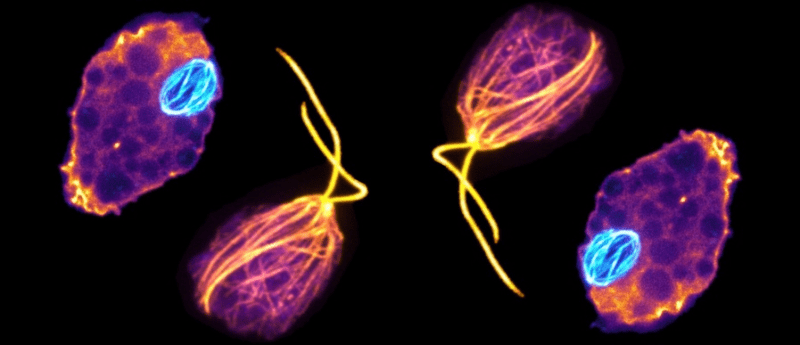
That relative is Naegleria gruberi, a single-celled species of Naegleria that is something of an amoebic shapeshifter. Diverging from the yeast-to-human lineage more than a billion years ago, Naegleria lack the cytoskeleton common to eukaryotic cells when in their amoeba form. Instead Naegleria assemble and disassemble microtubules from tubulin proteins to undergo mitosis and to temporarily differentiate from a crawling amoeba to a swimming flagellate for a brief time, resuming their life as a crawling amoeba within 2 to 300 minutes.
During mitosis, Naegleria express three additional evolutionarily divergent mitotic tubulins in comparison to the typical α- and β-tubulins – a second mitotic α- and two mitotic β-tubulins. One pair of tubulins are exclusively used during mitosis, while flagellate tubulins are utilized for movement.
 Shapeshifting bacteria: filamentation allows invasion of multiple cells
Shapeshifting bacteria: filamentation allows invasion of multiple cells
Scientists find a new species of bacteria that shapeshifts into a long thread-like form to invade its roundworm host, a technique that has not been observed before.
Researchers at University of Massachusetts’s Amherst’s Institute for the Applied Life Sciences (MA, USA), analyzed these different microtubule structures using high-resolution 3D photographs, then searched the Naegleria gruberi genome for α- and β-tubulins, identifying 13 α- and 9 β-tubulin genes. Some of these were closely related to those found in other eukaryotes, but others diverged wildly. They then reconstructed a phylogenetic tree of the α- and β-tubulins, finally arriving at two clades – Naegleria mitotic and flagellar tubulins branching into distinct familial tubulin clades.
The sub-clades that were most closely related to animal and fungal tubulins included those expressed during flagellate differentiation, which represented the majority of cytoplasmic tubulin proteins present in flagellates. These tubulin proteins and are not typically expressed in amoebae, and flagellate α-tubulins shar a relatively high percentage range of identicality with human α-tubulin A1B and β-tubulin B1.
The second sub-clades diverged more prominently, with the α-tubulins containing two sequences from both Naegleria gruberi and Naegleria fowleri, and an additional sequence from Acrasis kona and Stachyamoeba lipophore. In these second-clade sequences, the α and β-tubulins were both slightly below 60% identical to human α-tubulin A1B and β-tubulin B1.
The implications of this discovery are far reaching. Practical potentialities include treatments for brain-eating infections while theoretically, the findings could help explain the processes of evolutionary diversity and complexity, with Naegleria’s distance from both fungi and fauna making it a good jumping-off point for theories concerning the last common eukaryotic ancestor.
In essence, we’re being taught about our existence by a single-celled organism that’s happy to eat the very object that allows us to understand our existence in the first place.