Shapeshifting bacteria: filamentation allows invasion of multiple cells
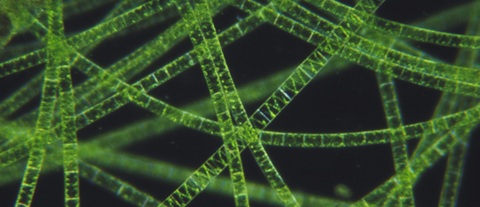
A new species of bacteria shapeshifts into a long thread-like form in a never-before-seen technique to spread from cell-to-cell within its roundworm host.
A research team from San Diego State University (CA, USA), identify how a new species of bacteria invades the roundworm using a shapeshifting mechanism. Fittingly named after the Greek Fate Atropos (responsible for cutting the threads of life), Bordetella atropi takes on a thread-like form to invade multiple host intestinal epithelial cells. The mechanism may provide insight to how some pathogens infect and spread in humans.
Much like something out of science fiction, B. atropi grows up to 100- times the size of an average bacterium over the course of 30 hours without dividing – a process known as filamentation. Although similar sounding to the Hollywood tropes of bacterial transmission via the likes of zombie bites, B. atropi luckily only spreads through this shapeshifting mechanism within the roundworm host and cannot infect humans.

Live fast, die young: bacterial fitness affected by growth rate
Research suggests rapid growth of bacteria can result in reduced fitness when food sources run now.
Read why this might inform antibiotic administration here.
The study, led by Tuan Tran, found that B. atropi undergoes a unique form of filamentation when in a nutrient-rich environment to enable the bacterium to grow continuously in an invasive manner. This was an unusual finding as other bacteria tend to undergo filamentation in response to DNA damage or environmental stressors; this typical response prevents bacteria from dividing until the cell damage is fixed.
In fact, the team demonstrated that B. atropi utilizes this shapeshifting pathway in order to spread from cell-to-cell within the roundworm host, rather than in response to stress. As the bacteria infect the roundworm host cells and detect the nutrient rich environment, they switch on the filamentation pathway, enabling them to quickly infect more host cells and gain access to further nutrients for growth.
“We went from finding the worm in the ground, finding the bacteria, and carrying it all the way to the molecular mechanism of how the bacteria infects the worm,” said Robert Luallen, senior author of the study. “We’re seeing things that no one’s ever seen before.”
Despite B. atropi and the roundworm not being able to infect humans, the use of filamentation in this way may pose a threat when utilized by human bacterial pathogens. Likewise, the nutrient-induced filamentation process may be used by other bacteria to form biofilms which are notoriously problematic in hospital settings, causing issues such as coating the tubing of catheters, leading to complications for patients.