A rapid FIRE method for epigenome editing

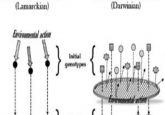
To better understand the role played by chromatin regulatory complexes in the epigenetic regulation of gene expression, researchers developed a rapid and reversible technique for editing the epigenome.

A key goal for researchers studying epigenomics is understanding how particular epigenetic marks, such as DNA methylation or histone modification, control gene expression. These marks are made, read, and removed by enzymes in large, multiprotein regulatory complexes that bind chromatin. To study how these chromatin regulators function, researchers need new methods, since existing approaches, such as in vitro chromatin templates, are inadequate.
Now, a team led by Gerald Crabtree from Stanford University has developed a method to rapidly and reversibly edit the epigenome in mammalian cell lines by recruiting endogenous chromatin complexes to any genomic locus.
The researchers targeted a catalytically dead mutant of Cas9 (dCas9)—containing the appropriate single-guide RNA (sgRNA)—to bind to the desired genomic location and serve as an anchor for the subsequent inducible, indirect binding of the endogenous chromatin regulatory complex of interest. They accomplished this by taking a single subunit in the chromatin complex and fusing it with the Frb (FKBP-rapamycin-binding domain of mTOR) tag. In the presence of the small-molecule dimerizer rapamycin, the Frb tag rapidly dimerizes with its partner protein domain Fkbp (FK506-binding-protein) that is fused to an MS2 bacteriophage coat protein, which in turn, binds to MS2 RNA hairpin loops fused to the sgRNA in the dCas9, thus bringing the chromatin complex to the targeted genomic locus.
Crabtree’s group used their method, named Fkbp/Frb inducible recruitment for epigenome editing by Cas9 (FIRE–Cas9), to recruit a heterochromatic complex to sites upstream of a gene in human embryonic kidney (HEK 293) cells and another gene in mouse embryonic stem cells (mESCs). This caused the appropriate repressive histone methylation mark to be written at those genomic loci, reducing mRNA expression from both genes. The addition of FK506, a competitive inhibitor of RAP that binds only to Fkbp and prevents its dimerization with Frb, reversed the recruitment of the complex. This resulted in the erasure of the repressive epigenetic mark at the sites of recruitment and the restoration of mRNA expression, demonstrating the reversibility of the method.