Fat’s forces affect distant tissues
Fat-derived microRNAs create systemic effects by regulating other organs, but a shortage contributes to disease.

Adipocytes after differentiation
Credit: Metabolic Systems Biology lab, UC San Diego Jacobs School of Engineering
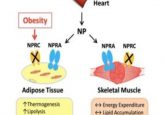
Body fat has important physiological roles such as energy storage and hormone signaling. It also regulates metabolism via the release of special cytokines known as adipokines. Now, a Nature study shows how a new class of adipokine—fat-derived circulating microRNAs (miRNAs)—affects distant body tissues and may contribute to systemic diseases such as obesity.
“We were looking for novel mechanisms of communication between fat and other tissues and thought about miRNAs based on our previous study where they changed in adipose tissue with aging,” said senior author Ronald Kahn at Joslin Diabetes Center. “In theory, they have potential to regulate multiple processes in all tissues of the body.”
miRNAs can regulate other protein-producing RNAs and travel throughout the body via the circulation. Hence, “It was important to focus on exosomes since they are a specialized fraction of circulating miRNAs that might be most likely to get into other tissues,” explained Kahn.
To pinpoint the role of fat-associated miRNAs, Kahn and colleagues at Harvard Medical School bred mice lacking the miRNA-processing enzyme Dicer in adipose tissue. The resulting mice had substantially decreased levels of exosomal miRNAs, which the researchers could restore by transplanting normal fat into the mice.
To see if human fat has the same capability, Kahn’s team profiled exosomal miRNA in serum from human patients with and without lipodystrophy—a condition involving fat tissue-degeneration. Those with lipodystrophy had fewer circulating miRNAs than expected.
Further analysis showed that exosomal miRNAs altered the expression of a liver gene, Fgf21, that increases lipodystrophy, suggesting that fat might send signals via miRNA to the liver to modulate lipodystrophy.
“These observations would definitively confer to miRNA an endocrine function and add evidence to the well-established roles of exosomes in miRNA distribution to peripheral tissues,” commented research director Philippe Lefebvre at INSERM, who was not involved in this study.
Kahn suspects that miRNA levels vary in people with diseases affecting fat mass (e.g. HIV-associated lipodystrophy and obesity) or fat distribution and function (e.g. diabetes and aging). Future work may translate this discovery into new methods for diagnosing and treating metabolic diseases.