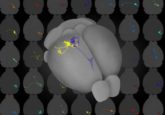
Identity crisis of the taste buds
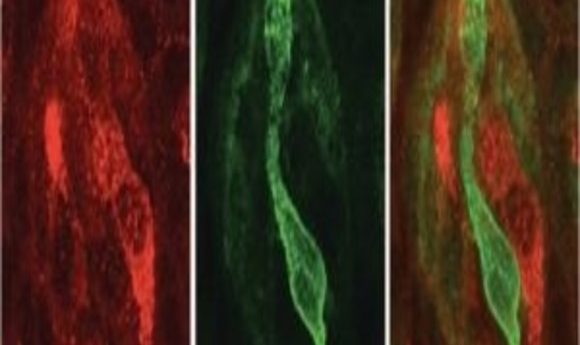
Distinguishing between bitter and sweet depends on the expression of two key connectivity molecules.
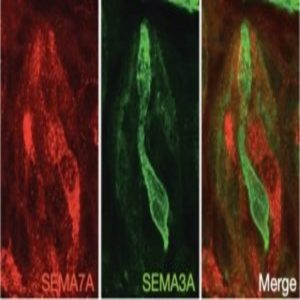
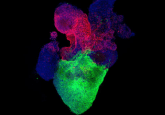
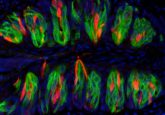
An example of sweet (red) and bitter (green) taste receptor cells on a taste bud. SEMA7A (red) is a key connectivity molecule for maintaining the connection between sweet specific cells and sweet specific relay neurons, whereas SEMA3A is a key connectivity molecule connecting bitter specific cells to bitter specific relay neurons (1).
Imagine biting into dessert, and instead of it tasting sweet, it tastes bitter. The ability to differentiate between bitter and sweet is essential not only for enjoying food, but also for survival, as bitter tastes are often associated with toxins. The tongue accomplishes this feat using hundreds of tiny bumps, each containing a single taste bud that houses hundreds of taste receptor cells, each dedicated to detecting a single taste: either sweet, salty, sour, bitter, or savory.
Taste receptor cells connect through the taste bud to specific relay ganglion neurons, which signal the difference between flavors to the brain. For example, sweet-specific taste receptor cells connect to sweet-specific neurons and bitter-specific taste receptor cells connect to bitter-specific neurons.
Taste receptor cells live only 5-20 days—or until you drink something extremely hot or ravenously bite your tongue. So, how is the connection between ephemeral taste receptor cells and their corresponding permanent neurons maintained? A recent study published in Nature shows for the first time that connectivity molecules are key to maintaining consistent wiring and preventing the confusion of taste.
“I’m amazed by the fact that if you give a young toddler lemon, which is very sour, you would see his response. We can even empathize with what the child is going through because the behaviors that are caused by tastants are very stereotypical,” said lead author Hojoon Lee, a postdoctoral researcher in Charles Zuker’s lab at Columbia University.
Lee and colleagues chose to investigate only bitter and sweet tastes. They isolated bitter and sweet taste receptor cells from mice using fluorescent markers. “I thought there can’t be a way where sweet neurons connect to bitter taste receptor cells, otherwise the mouse is going to be in big trouble,” said Lee, referring to toxins that taste bitter. “So, I picked those two populations because I thought these were probably never going to be cross wired, so there should be a strong signal that safe-guards against that.”
The team performed RNA-sequencing on the isolated taste receptor cells and searched for candidate proteins that might play a role in maintaining the taste receptor cell and neuron connection. They found that semaphorin 3A (SEMA3A), a connectivity molecule, was enriched more than 100-fold in bitter taste receptor cells compared to other cell types. Likewise, SEMA7A was more common in sweet taste receptor cells.
They next depleted SEMA3A to test its role in bitter specific cells and assessed the specificity of the connection between ganglion neurons and their corresponding taste receptor cells using in vivo functional calcium imagining.
“People have looked at wiring, but we’ve never really had a platform for looking at the identity of single neurons and how they are wired,” said Lee. In vivo calcium imagining allowed the researchers to record responses of specific ganglion neurons to different tastes placed on a mouse’s tongue. When SEMA3A was depleted, they observed a reduced number of bitter specific neurons and an increase in the number of neurons that were activated by more than one taste.
The researchers then engineered a mouse line that expressed SEMA3A in sweet-specific cells and simultaneously depleted SEMA3A in bitter-specific cells. As predicted, bitter-specific neurons responded to sweet-specific cells, and there was a significant increase in the population of bitter-sweet doubly tuned neurons. Lee’s team concluded that SEMA3A most likely acted as an attractor molecule, recruiting bitter-specific relay neurons to sweet taste receptor cells.
“To me, what was unsatisfactory is that by removing SEMA3A from bitter cells and then mis-expressing it in sweet cells, we still see about 30% of neurons going correctly to bitter still. So, this strongly suggests that there are other factors,” said Lee who intends to investigate other possible candidate molecules that emerged during their RNA-sequencing analysis. There are also likely other key connectivity molecules specific for each of the other tastes still remaining to be discovered.