To survive hard times, a plant degrades a part of itself
About 20 years ago, a leader in plant science laid the foundation for a floral hormone signaling system. Now, her former students have discovered the molecular mechanisms that underpin her discoveries.
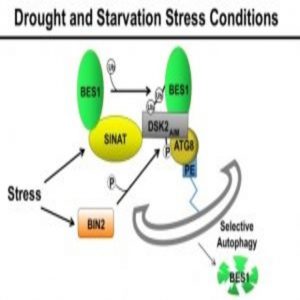
In a pair of studies, researchers determine how degradation of a BR protein helps balance plant growth in times of stress – ultimately helping the plant survive.
Credit: Yanhai Yin and Trevor Nolan of Iowa State University.
In the world of plant science, Joanne Chory is a pioneer. She’s known for discovering the roles of growth-promoting hormones, called brassinosteroids (BR), and mapping out the BR signaling system. Eighteen years ago, Yanhai Yin joined Chory’s lab as a postdoctoral fellow with the goal of understanding how plants use the BR pathway to control their growth and responses to stresses such as drought or starvation. When he left her lab, Chory offered Yin a nice parting gift: yeast two-hybrid screens, which remained in a freezer box for 10 years.
Today, Yin is a professor of genetics, development and cell biology at Iowa State University. About three years ago, Yin’s Ph.D. student, Trevor Nolan, decided to revisit those long-forgotten yeast two-hybrid samples, which turned out to contain a treasure trove of information. With the help of colleagues in the U.S. and China, the team discovered that autophagy—a cell’s molecular recycling system—plays a key role in balancing plant growth in times of stress (1).
“We kind of struck gold,” Yin said. “In the last year or so, we made several important findings.”
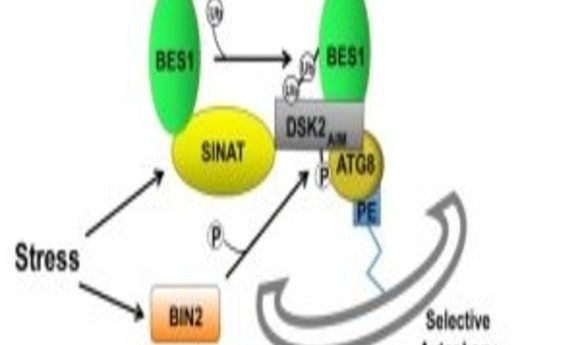
The team first reported a key interaction between the BR pathway and drought response a few months ago when they described how two transcription factors, BES1 and RD26, control a plant’s response to drought. By digging a little deeper, the group now reports in the journal Developmental Cell that BES1, a critical signaling protein of the BR pathway, isn’t only inhibited by a certain stress response. It’s also degraded through selective autophagy, which targets specific organelles and cell parts.
In this experiment, researchers used drought and carbon starvation to instigate change in the plant Arabidopsis thaliana, or thale cress. They noticed a protein called BIN2, which starts the chain of degradation, and plays a key role in phosphorylating a protein receptor called DSK2 which specifically recruits BES1 to the autophagy pathway and commences degradation.
“This work helps us appreciate that selective autophagy may be very important in terms of signaling as well as degrading,” said Nolan, lead author of the study. “At least to my knowledge, this is the first example in plant systems that shows selective autophagy regulating hormone signaling.”
Alongside this research, a team led by another former postdoctoral fellow of Chory’s published a companion study in Developmental Cell, where they revealed the importance of light-mediated plant growth and development in relation to BES1 degradation (2). A class of proteins called SINATs activate when plants starve from lack of sunlight, and they ubiquitinate BES1 to prompt the cell to break it down.
These new observations add to the greater story of how autophagy fundamentally works, how light promotes growth and development, and how flora can potentially withstand changing climates.
“The end-result of all these processes is to shut down the BES1 function, but the way these mechanisms operate to balance plant growth is completely different,” Nolan explained. “They work in concert, and that’s what we need to better understand.”