Tracking the causal genetics of Fragile X
Researchers report a single protein underlying the cellular and behavioral symptoms associated with Fragile X syndrome, one of the leading known causes of Autism Spectrum Disorder.
![]()
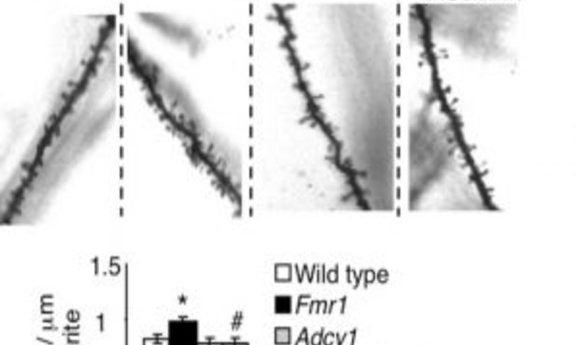
Genetic reduction of Adcy1 corrects the higher dendritic spine density in a mouse model of Fragile X syndrome.
Credit: Hongbing Wang & Michigan State University
According to a new study, researchers have identified a single protein they believe is directly responsible for driving Fragile X syndrome (FXS), the most common single-gene cause of Autism Spectrum Disorder (ASD). The findings, published in Nature Communications, demonstrate how manipulation of the gene offset FXS-related symptoms, such as increased neuronal signaling and autism-like behavioral patterns, in mice
“Autism can have many different causes that display similar symptoms, and within that, there are syndromic autisms like Fragile X that are caused by a problem in a single gene,” said Hongbing Wang, who co-authored the study at Michigan State University.
Of 600–3000 potential Fragile X mental retardation protein (FMRP) targets suggested by previous whole-genome screening studies, Wang’s lab identified type 1 adenylate cyclase (ADCY1) as a functionally relevant molecule. Lab tests revealed that in the hippocampus of FXS mice, the level of ADCY1 protein was increased by up to 25% compared with typical wild-type mice.
To investigate, Wang’s team deleted the ADCY1 gene in Fragile X mice and observed that patterns of abnormally elevated neuronal signaling and autism-related behaviors changed to resemble those seen in the control mouse group. The team also observed corrective changes to the physical appearance of Fragile X neurons, which are typically characterized by excessive dendritic, in the mice spines.
“Dendritic spines contain neural transmitter receptors important for synaptic function, and higher spine density is a prominent developmental problem in FXS patients,” Wang explained. “Strikingly, when we manipulated this single target, we saw correction of these Fragile X and autism-related phenotypes.”
Next, the researchers injected the FSX mice with a compound called NB001, which crosses the blood-brain barrier to inhibit ADCY1 activity in FSX neurons. As a result, the team observed normal levels of protein translation, spine density, and behavior.
“There is great potential, but still many steps to confirm what we have found with mice in humans,” said Wang. “If we can identify ADCY1-inhibiting compounds from the available FDA-approved drugs with reasonable safety and pharmacokinetics profile, it may save time in terms of drug development.”