Within ARMMs reach
A new study reveals a non-canonical cell signaling pathway orchestrated by a novel extraceullar vesicle.
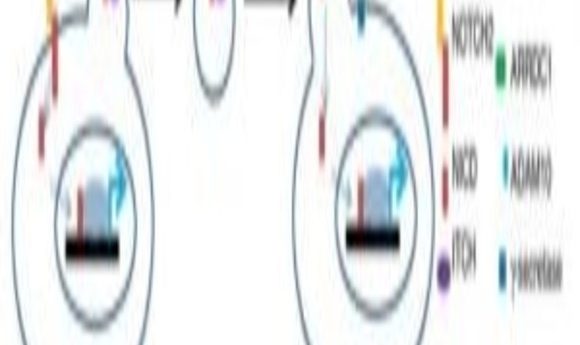
Proposed model for ARMMs-mediated non-canonical NOTCH2 signaling (1).
Cells release important molecules such as proteins and RNAs into the extracellular environment via tiny packages called extracellular vesicles (EVs). Most studies exploring EV trafficking focus on multivesicular bodies (MVBs), which are shuttled to the plasma membrane by lysosomes prior to their release via exocytosis. Nearby cells receive these packages, initiating a signaling cascade.
Quan Lu from Harvard University previously reported on a novel type of extracellular vesicle known as the arrestin domain-containing protein 1 (ARRDC1)-mediated microvesicle (ARMM) that, unlike MVBs, is produced directly at the plasma membrane and requires arrestin-domain containing protein 1 (ARRDC1) for budding. However, the function of ARMMs has remained a mystery. Now, in Nature Communications, Lu and his colleague Qiyu Wang reveal how ARMMs mediate an alternative signaling route that enables long-distance communication between cells (1).
Given their unique localization at the plasma membrane, Lu hypothesized that ARMMs may transport plasma membrane-associated molecules, such as receptors, to nearby cells. To test this hypothesis, the researchers explored the molecular components of EVs using cells expressing a GFP-tagged ARRDC1 protein. Using mass spectrometry, they identified over 100 proteins enriched in the ARRDC1-GFP cells, one-third of which are plasma membrane proteins.
Among these plasma membrane proteins, the researchers identified both NOTCH2 receptors and related proteins. “If you find multiple components of the NOTCH pathway, then that tells you something,” said Lu.
NOTCH receptors mediate a variety of physiological functions via direct contact with neighboring cells. To test whether NOTCH2 receptors secreted into ARMMs transport to nearby cells, the researchers expressed GFP-tagged NOTCH2 in one group of cells and then separated them from a group of recipient cells using a membrane with pores just large enough to allow EVs to travel through.
Canonical NOTCH signaling depends on direct contact between the donor and recipient cells, but Lu’s team found that, with the help of ARMMs, NOTCH2 receptors transported across a distance to communicate with cells beyond their nearby milieu.
“The way that these vesicles carry NOTCH suggests to us that they could be engineered to carry therapeutic cargo,” said Lu. “For example, you can imagine that you can replace NOTCH with proteins for tumor suppression or for immune function.”
Submit Your Research to the F1000Research Cell & Molecular Biology Gateway
Contribute to advancing molecular biology by publishing your research with the F1000Research Cell & Molecular Biology Gateway. With trusted publishing, open access, and transparent peer review, your work will uphold the highest standards of rigor and integrity while driving innovation in cellular and molecular science.
Join a platform that values transparency, openness, and author control. Submit your research today at F1000Research Cell & Molecular Biology Gateway.