Validation of antibody tests to fight COVID-19 firmly underway
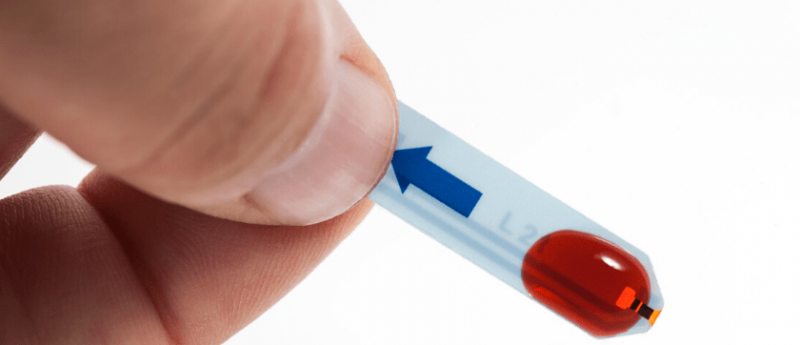
Two UK-based companies have been tasked with developing lateral flow immunoassay tests – antibody tests – for use as rapid diagnostic tools for the novel coronavirus disease, COVID-19.
The companies, Mologic (Bedfordshire, UK) and SureScreen (Derby, UK) are manufacturing as many test kits as possible to be able to meet the UK’s target of testing 25,000 people per day for coronavirus. Mologic has received a £1 million grant from the UK Government to help speed research and reach demand.
The antibody tests use gold nanoparticles in the test strip that detect COVID-19 biomarkers, IgG and IgM, which are released on interaction with antibodies embedded in the strip. As a sample – in this case a blood sample from a finger-prick is most likely used – moves along the strip the biomarkers come into contact with the antibodies and illicit a colour change, visible in a test line, in much the same way as you would see in a pregnancy test.
The test kit manufactured by SureScreen detects both IgM, a generalized infection biomarker, and IgG, a more specific SARS-CoV-2 biomarker, to 97.8% and 99.6% accuracy respectively and it does this within 10 minutes. The antibody tests are able to detect infection where the patients may be asymptomatic or where currently used PCR tests may give false-negative results. Having such accurate and rapid tests could help enormously in preventing the spread of infection with SARS-CoV-2 as earlier detection and isolation will reduce the risk of passing on the virus.
The antibody tests are able to detect infection where the patients may be asymptomatic or where currently used PCR tests may give false-negative results.
Mologic test kits are currently undergoing manufacture and optimization and will be available after rigorous validation tests. Global partners will be provided with the test kit once these validation tests have been completed, as well as being shipped to low-income countries at cost price to ensure global access to the technology. For more information on this see the full article on our sister site, Bioanalysis Zone here.
“Completion of the first prototypes is a significant step in Mologic’s development of a rapid diagnostic test for COVID-19 and we are proud of our team’s achievement in reaching this point so quickly, while maintaining the most rigorous standards. Diagnostics are a critical weapon in the fight against this pandemic and, once ready, this test will enable affordable, more accurate and earlier diagnosis of infection, limiting the spread of the disease.” Commented Paul Davis, Co-Founder and Chief Scientific Officer of Mologic.
By creating antibody tests that do not require laboratory facilities and specialized training, patients can test themselves at home and follow the correct procedures depending on the outcome. This will help to ease the burden on healthcare services across the world as they work to fight the pandemic.
For more free COVID-19 related content please visit our dedicated COVID-19 Hub on Infectious Diseases Hub here.
For more related news from The Nanomed Zone be sure to sign up here.