Cancer linked to DNA repair process
A DNA repair process, DNA charge transport, has been implicated in some cancers.
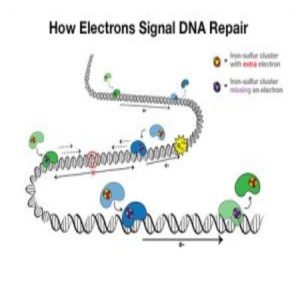
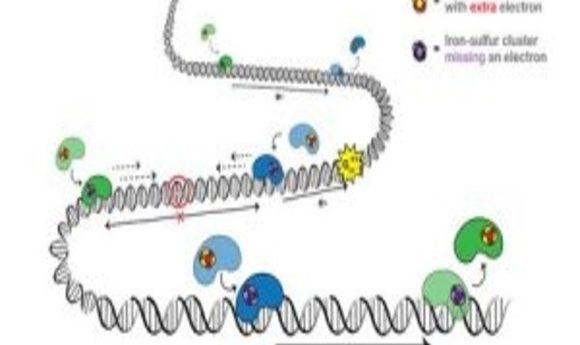
An electron travels from one protein (blue) to the next (green) and adds to the protein’s iron-sulfur cluster. With the extra electron, the green protein falls off the DNA, signaling that there is no DNA damage. If there is DNA damage, the electron won’t make it to a protein’s iron-sulfur cluster, so that the protein stays bound to DNA and moves toward the damage to fix it. Credit: Barton Lab/Liz O’Brien/Caltech.
DNA charge transport is a process, first discovered in the 1990s, that is utilized in DNA repair. Electrons can travel along DNA between DNA-bound repair proteins, signaling for these proteins to fix any DNA that is damaged.
It is a known process in bacterial cells; however, it has only recently been discovered to also occur in human cells with potential cancer-causing implications. The new study, published in Nature Chemistry, provides evidence linking a mutation in repair protein MUTYH, which is present in numerous cancer patients, to the DNA charge transport process.
A team from the California Institute of Technology (CA, USA) started looking into the potential link when they were contacted by researchers at the University of Southern California Norris Comprehensive Cancer Center (CA, USA) who had identified the unusual mutation.
“We have found that a mutation to a DNA repair protein associated with cancer can disrupt electron transport through DNA,” explains Jacqueline Barton, co-author of the study and chair of the Division of Chemistry and Chemical Engineering at California Institute of Technology (CA, USA).
“The work provides a strategy for thinking about how to possibly stabilize these repair proteins and restore their ability to carry out long-range signaling through DNA, so that the repair proteins can find and fix the mutations in DNA before they lead to cancer.”
Mutations in the MUTYH protein had been previously linked to cancer but this was the first mutation to be associated with the iron-sulfur cluster in the protein. This is important because the iron-sulfur clusters are the source of electrons in the repair proteins. On addition of an electron, the proteins lose affinity for the DNA and drop off, and vice-versa, on loss of an electron, the protein’s affinity for DNA increases.
The implication of the mutation is that the iron-sulfur cluster is degraded. Without this indication of a need for DNA repair, the proteins will drop off the DNA and any potential DNA damage will not be repaired.
The researchers hope that this could provide useful in the future as diagnostics for cancer patients or personalized medicine.