Could BeeR help design targeted cancer therapeutics?

A recently identified bacterial protein, named BeeR, is contributing to the design of novel anticancer drug delivery systems.
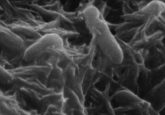
Researchers from King’s College London (UK) and the University of Washington (WA, USA) have discovered a previously unknown actin-like protein in a family of bacteria found in microbiomes of the human gut and soil. This protein’s unique structure has the potential to aid drug delivery of cancer treatments to the sites of tumors.
Actin is an abundant cellular protein in eukaryotes that can bind other actin molecules in the presence of ATP to form long spiral chains called filaments. Actin can also break down ATP, which causes the filaments to disassemble. These filaments have important functions in the outer membranes of cells, providing structural integrity as well as the ability to move and divide.
Bacteria contain proteins like actin that also form filaments in the presence of ATP and help the cell maintain its shape and divide. However, interactions between these filamentous proteins can vary widely and lead to dramatic differences in the structure of filaments, presenting an intriguing group of proteins to explore that could have a variety of applications in drug development.
 CRISPR for cancer: targeting cancer-specific genes in solid tumors
CRISPR for cancer: targeting cancer-specific genes in solid tumors
Targeted CRISPR lipid nanoparticles eliminated 50% of tumors in mice with head and neck cancer.
Setting out to investigate these proteins, the team conducted metagenomic analyses to identify actin-like proteins that adopt unique filament architectures. This screen revealed a protein – which they named BeeR – that is highly conserved in the Verrucomicrobiota phylum, a family of anaerobic bacteria found in soil samples and the gut microbiome. The team confirmed BeeR’s ability to polymerize in the presence of ATP – but not ADP – in a pelleting assay before using negative-strain electron microscopy to determine the length and ordering of the train-track-like filaments.
However, the real breakthrough was made after the team conducted cryo-electron microscopy, which revealed its structure; the BeeR filament adopts a three-stranded, parallel, staggered filament architecture with a diameter significantly larger than most other actin-like proteins and includes a cavity at its core.
“At this time, we don’t know the function of BeeR,” commented lead researcher Julien Bergeron. “Nonetheless, the identification of an actin-like protein forming a tubular structure transforms our understanding of the evolution of this critically important family of proteins.”
Now, Bergeron – via his spinout company Prosemble (London, UK) – is leveraging these findings to exploit the unique structure of the hollow BeeR tubes to create protein nanoparticles for targeted anticancer-drug delivery to tumor sites, already testing the nanoparticles in pre-clinical breast cancer models.
“Not only are the BeeR structures tubular, but they also have a cavity at their centre that is big enough to contain drug molecules. Since we can easily control the assembly and disassembly of the tube with ATP, it gives us a simple method to deliver and release the drugs at the desired location,” concluded Bergeron.