Novel tau therapies show promise for Alzheimer’s
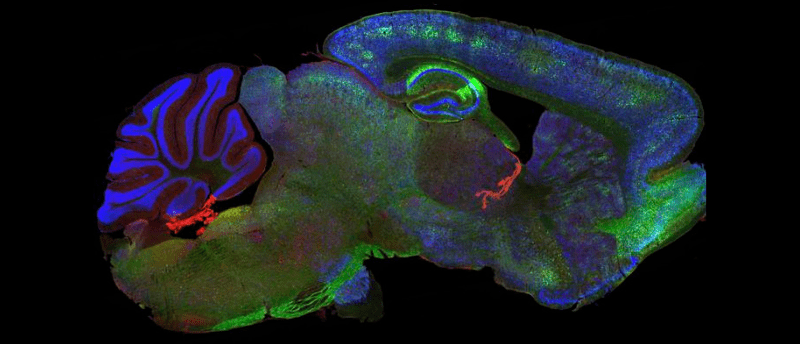
Two new therapies have successfully cleared tau aggregates in preclinical and mouse models, showing potential as dementia treatments.
Researchers at the Medical Research Council Laboratory of Molecular Biology (MRC LMB; Cambridge, UK) and the UK Dementia Research Institute (UK DRI) at the University of Cambridge (UK) have developed two novel therapies targeting tau aggregates, which accumulate in the brains of people with different forms of dementia, like Alzheimer’s. In two new papers, the researchers showed that the drugs successfully cleared aggregated tau from neurons, while leaving healthy tau, and also slowed the progression of neurodegeneration symptoms.
Tau and amyloid are the two main proteins that misfold and accumulate into aggregates in the brains of people with Alzheimer’s disease. Amyloid aggregates accumulate in the spaces between neurons so can be targeted with drugs such as monoclonal antibodies. Tau ‘tangles’, on the other hand, form inside neurons, making them more difficult to target. While there are drugs in development that target tau inside cells, such as anti-sense oligonucleotides, they act on all tau in the brain, including healthy tau, which helps provide structural support to neurons. This could therefore have a detrimental effect on the brain.
To address this, the researchers built on previous work, from co-leader of the study Leo James (MRC LMB), on the protein TRIM21, which detects antibody-bound viruses when they enter cells, tagging them for destruction by the proteasome. They demonstrated that by switching out virus-binding antibodies for tau-binding antibodies, TRIM21 would tag tau aggregates to be destroyed by the proteasome.
TRIM21 has a key feature: a part of the protein called RING, which is activated only when two or more TRIM21 proteins cluster together. This means that it only activates and tags its target for destruction when TRIM21 proteins are bound to adjacent, aggregated tau proteins. In the current project, the team utilized this feature to create two new therapies targeting tau aggregates.
 Connecting the dots: tau, astrocytes and neurodegeneration
Connecting the dots: tau, astrocytes and neurodegeneration
In this interview, Kathryn Bowles discusses her new project to investigate how the buildup of tau affects astrocytes, the role of astrocytes in the development and progression of progressive supranuclear palsy, the techniques she uses to investigate tauopathies and her top tips for iPSC models.
The first therapy, called RING-nanobody, combines the RING domain of TRIM21 with a tau-binding nanobody [1]. The second therapy, called RING-Bait, combines the RING domain with a copy of the tau protein, which gets incorporated into tau aggregates, activating and causing the aggregate to be destroyed once multiple RING-Baits are added.
The researchers delivered DNA encoding the therapies into cells containing aggregated tau and found both therapies cleared the tangles while leaving healthy tau undamaged.
Different neurodegenerative diseases can have different types of misfolded tau. Therefore, the team tested the therapies on cells containing aggregated tau proteins from brain tissue donated by individuals with Alzheimer’s disease or progressive supranuclear palsy. They found that RING-Bait could prevent tau aggregation in both diseases.
“Neurodegenerative diseases can have tau proteins that misfold in many different ways, raising the possibility of needing a different treatment for every disease,” explained James. “A useful aspect of RING-Bait is because it is attached to a tau protein, it’s a universal Trojan horse that should be incorporated into different types of tau aggregates exactly like the cell’s own misfolding tau protein.”
The researchers then tested the drugs on elderly mice with tau aggregates, using an adeno-associated virus to deliver the DNA. They found a significant reduction in tau aggregates in the brain cells in the treated mice within just a few weeks. The progression of neurodegeneration symptoms in the mice given RING-Bait slowed, and they showed significantly better motor function.
The researchers stress that these therapies require further development before they can be tested in humans. They hope that they will be able to use the same RING-Bait approach to target other aggregated proteins in other diseases such as Parkinson’s.