Polymer shows promise in treating Huntington’s disease

A polymer has been developed to optimize the use of a peptide to treat Huntington’s disease in mice.
Huntington’s disease is a devastating condition with no cure or treatments to slow its progression. Now, researchers led by Nathan Gianneschi (Northwestern University, IL, USA) and Xin Qi (Case Western Reserve University, OH, USA) have developed a protein-like polymer that helps prolong an active peptide’s survival and delivers it to the brain. The polymer has shown promise in preventing protein aggregation in a mouse model of Huntington’s and could be a helpful tool in the delivery of active peptides in other diseases.
Huntington’s disease is a progressive neurodegenerative condition caused by mutations in the huntingtin (Htt) gene. The mutant huntingtin protein (mtHtt) is misfolded, interacting abnormally with other proteins to form aggregates that accumulate in mitochondria and cause cell dysfunction and eventually cell death in the brain.
Previous research from Qi’s lab identified a protein, called valosin-containing protein (VCP), that binds to mtHtt, causing the protein to aggregate. The study also found a naturally occurring peptide that binds both VCP and mtHtt, preventing them from binding to each other and therefore preventing the formation of mtHtt aggregates.
“Qi’s team identified a peptide that comes from the mutant protein itself and basically controls the protein-protein interface,” Gianneschi explained. “That peptide inhibited mitochondrial death, so it showed promise.”
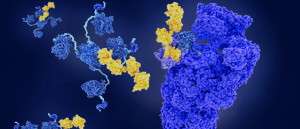 Protein degradation in high definition
Protein degradation in high definition
Protein-degrader structure and function have been captured in unprecedented detail.
However, peptides often can’t penetrate cells and are easily broken down by enzymes, so peptides themselves aren’t effective treatments. In addition, due to their small size, peptides can’t disrupt entire protein interfaces. To overcome this, the current team developed a biocompatible polymer with multiple copies of the active peptide. The structure features a polymer backbone with peptides attached like branches. The design protects the peptides from enzymes and helps them cross the blood–brain barrier and enter neurons.
The team then tested the polymer in a mouse model of Huntington’s. They found that the polymer stayed in the body 2000 times longer than just the peptide and prevented mitochondrial damage, preserving the health of neurons. The researchers also reported that the treated mice lived longer than controls, and their behavior reflected that of the control mice.
Gianneschi and the team plan to further optimize the polymer, with Gianneschi having a personal connection to the disease. “My childhood friend was diagnosed with Huntington’s at age 18 through a genetic test,” Gianneschi said. “He’s now in an assisted living facility because he needs 24-hour, full-time care. I remain highly motivated — both personally and scientifically — to continue traveling down the path.”