Protein degradation in high definition
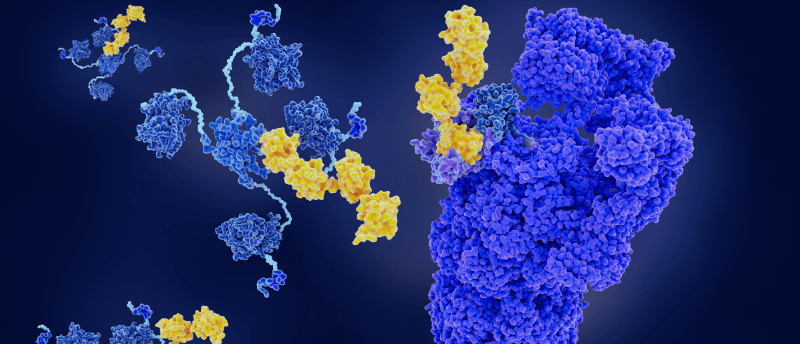
Protein-degrader structure and function have been captured in unprecedented detail.
Protein degraders are recognized as an exciting avenue for drug discovery and development, providing targeted therapies for ‘undruggable’ diseases. Now, researchers at the University of Dundee (UK) have revealed previously invisible levels of detail into degrader-mediated protein ubiquitinability, providing more information about how protein degraders function and the potential for more effectively targeted drugs.
Proteins that aren’t working properly – which may be disease-causing – need to be tagged with a small regulatory protein called ubiquitin so the protein-recycling systems in our cells are directed to destroy these problematic proteins. To do this, protein degraders capture the disease-causing protein and make it stick to the protein-recycling machinery in the cell, which then fires a ubiquitin bullet at a certain area of the protein. This is an exact process, requiring the protein degrader to position the target protein appropriately to allow ubiquitin to tag the protein in just the right place, known proverbially as the ‘bull’s eye’.
“Protein degrader molecules work in a way that is fundamentally different from the way conventional drugs work. However, until recently the exact details of how this process works at the molecular level had remained elusive. Proteins are typically a few nanometres large, which is 1 billionth of a metre, or 1 millionth of the width of a hair. So being able to ‘see’ them in action has not been possible, up until now,” explained Alessio Ciulli, senior author of the study.
Top tips for spike-in normalization
See a spike in your DNA–protein interaction quantification results with these guidelines for spike-in normalization.
Cryo-EM is a technique that flash-freezes proteins, utilizing an electron beam and high-resolution camera to generate millions of 2D images of proteins. The researchers used cryo-EM and AI models to generate 3D renderings of protein-degrader drugs in action. To investigate how degrader molecules – in this study MZ1, which was developed in Ciulli’s lab – tag disease-causing proteins, the team used high-end mass spectrometry.
They examined how MZ1 was able to hold onto target protein Brd4BD2 and direct it toward UBE2R1, a member of the ubiquitin-conjugating enzyme family, demonstrating the proficiency of MZ1 to position the target protein for degradation and the favorability of the target protein for ubiquitination by UBE2R1.
The importance of degraders for facilitating protein degradation efficiently and productively was demonstrated by the molecular insights provided by the team. The unprecedented detail revealed in the study contributes to a greater understanding of the degrader–complex structure and its functionality, which is information that researchers can use to develop more effective drugs.
Ciulli concluded, “This is incredibly exciting work and opens up the possibility of even more effectively targeted drugs able to finally treat some diseases which up until now have been too difficult to tackle.”